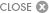Action required: Please refresh your browser
We have recently implemented some changes that require a hard refresh of your browser: Please hold down the CTRL-key and press the F5 key.
After a successful hard refresh, this message should not appear anymore.
More details about this topic are available here »
| Neuronoff Announces Safety and Functionality Success in First-in-Human Trial of Injectrode for Minimally Invasive Chronic Pain Relief (LIFE study) | ||
| By: PR Newswire Association LLC. - 23 Apr 2024 | Back to overview list |
|
|
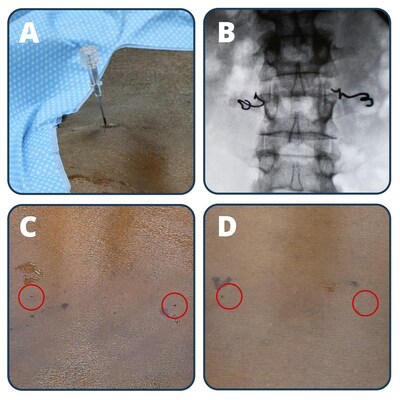
CLEVELAND, April 23, 2024 /PRNewswire/ -- Neuronoff, Inc., a pioneering medical device company dedicated to developing minimally invasive neuromodulation solutions, announced a significant milestone in its mission to transform chronic pain management. The company has successfully completed the first-in-human clinical trial evaluating the safety and effectiveness of its flagship product, the Injectrode, designed to treat chronic pain. Details of the Lumbar Injectrode Feasibility Evaluation, or LIFE study for short, can be found with the NCT06206356 trial identifier. The study, designed primarily as a safety trial with a secondary effectiveness measure, met all goals while providing valuable learnings for future studies. Each of the ten study participants received injectable electrodes (Injectrodes) placed unilaterally or bilaterally near the dorsal rami innervation to the erector spinae and multifidus muscles in the lower back for less than 30 days. Peripheral nerve stimulation of the dorsal rami may provide afferent input to the spinal circuitry in order to initiate neuromodulatory and rehabilitative treatment of non-radiating, axial, and facetogenic lower back pain. Simultaneously, efferent projections in the dorsal rami allow for immediate verification of successful stimulation by using evoked contractions of erector spinae musculature. The Injectrode placement was conducted by 18 gauge needle (no incisions) under local analgesia only at the level of the skin and subdermis. The placement location was determined using fluoroscopic visualization and confirmed by electrical test stimulation during the placement process. The trial demonstrated the feasibility of transcutaneous stimulation (voltages less than 30 V) immediately after placement and then again on day 25+/-3 of the study prior to device removal. This indicates the Injectrode's ability to deliver electrical neuromodulation at typical therapeutic levels from an external pulse generator across the skin all the way to the lumbar dorsal rami, driving the activation of all parts of the erector spinae (iliocostalis, longissimus and spinalis) as well as multifidus muscles as confirmed by ultrasound visualization, patient proprioception, and physician palpation. The device maintained a strong safety profile throughout the study with no serious adverse events and required no post-insertion analgesics or sutures. The study's Principal Investigator, Dr. Amol Soin, expressed his excitement about the implications of the findings, stating, "The Injectrode's consistent performance and ability to remain precisely on target throughout the study is a testament to its reliability and potential to transform neuromodulation therapies. During the trial, we observed consistent erector spinae musculature contractions visualized by ultrasound, confirming the device's effectiveness in delivering targeted stimulation. The ease of placement and removal, combined with its ability to provide precise stimulation, positions the Injectrode as a game-changer for patients seeking minimally invasive, drug-free options for chronic pain relief." Dr. Hesham Elsharkawy emphasized the Injectrode's potential impact on patient care, stating, "The Injectrode represents a paradigm shift in the field of neuromodulation. Its unique flexible design allows for optimal customization to the patient's anatomy and has the potential to be both a temporary and permanent solution without an internal pulse generator. Remarkably, the entire procedure takes just 5-10 minutes, making it highly efficient and accessible for patients seeking relief from chronic pain. The Injectrode is minimally invasive without compromise." Andrew Shoffstall, Chief Scientific Officer at Neuronoff, stated, "The study comprised a major leap forward for the development of the Injectrode, providing valuable information that builds upon the company's prior preclinical testing, including assessment of tolerability of sensory perception during stimulation, and first-in-human feasibility of the Injectrode's transcutaneous stimulation approach." "It was beautiful to see the trifecta of safety, effectiveness, and aesthetics be demonstrated so clearly," said Manfred Franke, CEO of Neuronoff. "Using an 18 gauge (1.3mm) diameter needle to implant the Injectrode means that a skin puncture remaining after placement is smaller than what is commonly seen during a blood plasma donation. This enabled the use of steri-strips instead of sutures at the injection site. While safety, effectiveness, and clinical usability criteria dominated the focus of this study, I believe that future patients and clinicians alike will appreciate that the injection locations had become essentially invisible in under four weeks. For me, aesthetic outcomes of a medical intervention represent a quality of care measure." Neuronoff plans to present more detailed findings from the LIFE study at upcoming medical conferences, showcasing the Injectrode's potential to transform the landscape of chronic pain management. For more information about Neuronoff and the groundbreaking Injectrode technology, please visit www.neuronoff.com. About Neuronoff Media Contact: Manfred Franke, manfred@neuronoff.com
SOURCE Neuronoff 
|
||
|
||
 | Back to overview list | |